Last Updated on October 9, 2024 by The Health Master
The US drug regulator has stepped up its inspection activity in India and plans to have more surprise or “short notice” inspections, ending the two-year reprieve from “warning letters” and other regulatory measures that Indian drug makers have enjoyed since the onset of the C-19 pandemic.
“In terms of (inspection) activity (in India), we are getting closer to pre-pandemic levels,” Sarah McMullen, country director-India at the US Food and Drug Administration (USFDA), said on the sidelines of a recent industry event in Mumbai.
McMullen said that the US regulator has resumed surprise inspections of pharma units in India.
India has the largest number of USFDA-registered drug manufacturing facilities outside of the US. The US accounted for 29% of the total pharma exports of India, worth $24.62 billion in FY22.
On average, during the pre-pandemic period, India used to see about 200 inspections annually. This number dropped to 80 in 2020, and to just 5 in 2021 due to C-19-related disruptions.
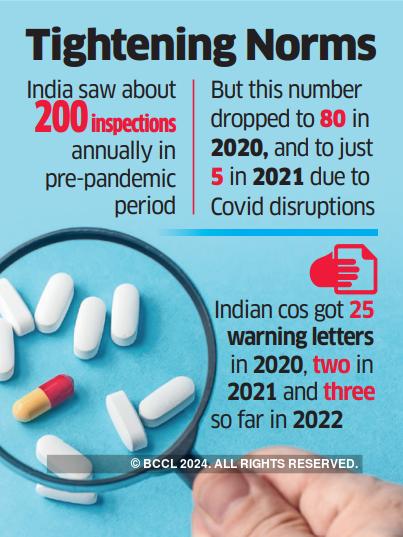
This resulted in official action indications (OAIs) and warning letters falling significantly. In 2020, Indian pharma companies received 25 warning letters. This number dropped to 2 in 2021 and stood at 3 in 2022.
The OAIs, which ranged anywhere from 40-50 a year in India, also fell steeply during the pandemic period. According to a Motilal Oswal report, Indian manufacturing sites have received 60 OAI citations from Sep 19-Sep to Sep 22.
The issue of both warning letters and OAI results in a lack of approval for the sale of new generic drugs to the US from the concerned plant, often resulting in a fall in the stock price of the impacted company. Postponed inspections also mean delays in new product approvals, hurting US businesses.
USFDA had to postpone foreign inspections or prioritize them based on mission-critical status. With the pandemic receding, the US agency plans to have hundreds of unannounced or short-notice inspections.
After raids in Pune, FDA cancels production license of Johnson and ohnson’s baby powder unit
USFDA issues Form 483 to Indoco with zero observation
NPPA fixes Retail Prices of 37 Formulations: September 2022
High Court: Marketing Companies will have a right to the Trademark
Govt is in final stage to cap margins of key medicines
PCI to amend Exit Examination Regulation for Diploma in Pharmacy
Drug alert: 45 out of 1330 samples declared as NSQ in August 2022
Panel recommends a separate legislation for Medical Devices
Govt adds 34 drugs in NLEM – National List of Essential Medicines
Latest Notifications regarding Pharmaceuticals








