- Possible mechanism to strengthen FDAs in regard to cognizable offences in the light of SC judgement - September 1, 2020
- Operation of Blood Storage Centres - January 4, 2020
- Risk Management of Medical Devices – ISO: 14971 - December 14, 2019
Last Updated on January 4, 2024 by The Health Master
Introduction:
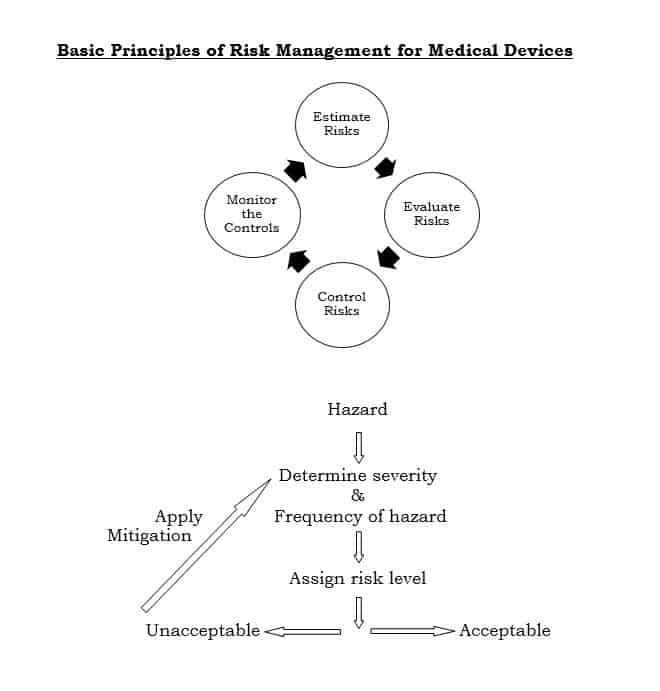
This international standard specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device and evaluate the risks associated with these hazards, control these risks and monitor effectiveness of that control.
ISO: 14971 involves following steps:
- General requirements for risk management
- Risk analysis
- Risk evaluation
- Risk control
- Evaluation of overall risk acceptability
- Risk management report
- Production and post-production information
What is Risk:-
Combination of probability of occurrence of harm and the severity of that harm
What is Risk Management:-
It is the systematic application of management policies, procedures and practices for analysis, controlling and monitoring risk.
Also read another article of this author “License for Manufacturing and Import of Medical Devices”. Click here
Why Risk Management:-
- Regulatory requirement.
- To avoid product recall.
- To avoid market complaint.
- To avoid damage of product, market and company image.
- To ensure safety of Medical Devices.
- Product liability – avoid compensation.
To explore more about Medical Devices, Click here
To download Medical Devices Rules 2017, Click here


