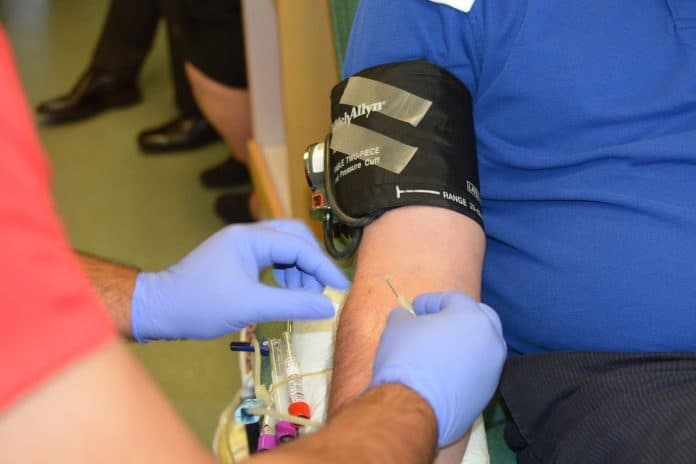
Last week, just as the US Food and Drug Administration was preparing to issue an emergency authorisation for blood plasma as a Covid-19 treatment, a group of top health officials, including Anthony Fauci, intervened, arguing that emerging data on the treatment was too weak, according to two senior officials.
The authorisation is on hold for now as more data is reviewed, according to H. Clifford Lane, the clinical director at the National Institute of Allergy and Infectious Diseases. An emergency approval could still be issued in the near future, he said.
Also read: Pharmacopoeial status of Blood and its components
Donated by people who have survived the disease, antibody-rich plasma is considered safe. President Trump has hailed it, but clinical trials have not proved whether plasma can help people fighting the coronavirus.
The Health Master is now on Telegram. For latest update on health and Pharmaceuticals, subscribe to The Health Master on Telegram.