Last Updated on January 8, 2024 by The Health Master
The government yesterday capped the trade margins of five critical medical devices like oximeter and digital thermometer, used extensively in the treatment and prevention of C-19 at 70 per cent to bring down prices.
The National Pharmaceuticals Pricing Authority (NPPA) has invoked extraordinary powers under the Paragraph 19 of DPCO, 2013, vide notification No. S.O. 2808(E) dt 13-07-2021 to put a cap on trade margin of five medical devices — oximeter, glucometer, BP monitor, nebuliser and digital thermometer.
“NPPA brings pulse oximeter, glucometer, BP monitor, nebuliser and digital thermometer under trade margin rationalisation. Caps Margin at Distributor @70%,” the drug pricing regulator said in a tweet yesterday.
The revised prices of the five medical devices would be effective from 20th July, it added. “Existing margins range from three per cent to 709 per cent across five categories,” NPPA tweeted.
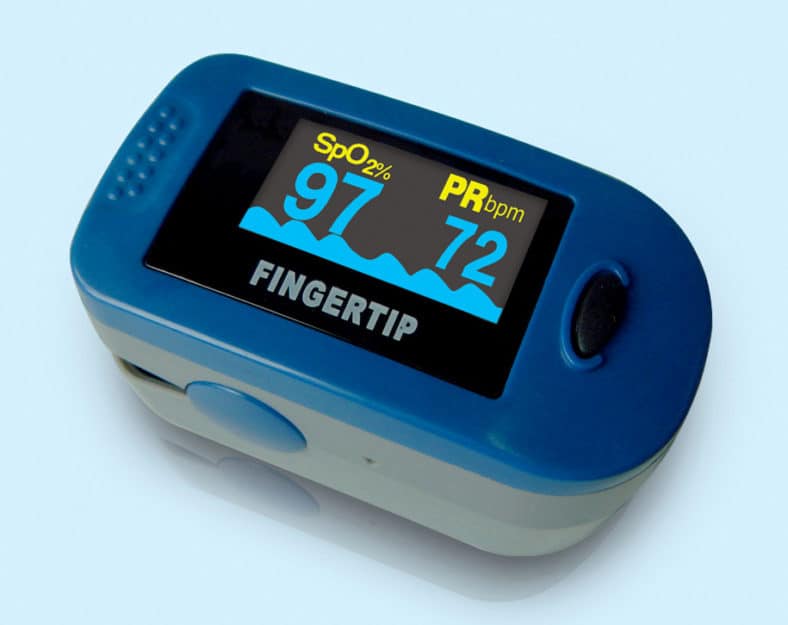
With an aim of making the medical devices affordable during the evolving situation of C-19 pandemic, it is felt necessary to regulate the trade margin on these medical devices, NPPA said in its order.
The authority noted that the data collected from the manufacturers/marketers/importers pointed out that the existing trade margins on the five medical devices ranged up to 709 per cent from price to distributor to MRP level.
Licensing procedure for Medical devices
Latest Notifications: Medical Devices
“Now, therefore, in the exercise of the powers vested by the government of India in the Ministry of Chemicals and Fertilizers, having been satisfied that in view of the extraordinary circumstances in public interest, as explained above, the government, hereby, invokes the provisions of Paragraph 19 of the DPCO, 2013 to regulate the price of these five medical devices,” NPPA stated.
By invoking Paragraph 19 of DPCO, 2013, the government hereby puts a cap on trade margin of pulse oximeter, blood pressure monitoring machine, nebuliser, digital thermometer, glucometer at first point of sale of product, it added.
The manufacturers of these five medical devices selling at price higher than the new MRP structure shall revise the prices downward, NPPA said.
“The manufacturers not complying with the MRP so computed..shall be liable to deposit the overcharged amount along with 15 per cent interest p.a. from the date of increase in price in addition to penalty upto 100 per cent of the overcharged amount under the provisions of the Drugs (Prices Control) Order, 2013,” it stated.
The Paragraph 19 of DPCO, 2013, authorises the NPPA to control the prices of drugs that are not under the National List of Essential Medicines (NLEM) under extraordinary circumstances in the public interest.
Last month, the government capped the trade margin on oxygen concentrators at 70 per cent by invoking extraordinary powers under Para 19 of the DPCO, 2013.
Latest notifications – DPCO /NPPA
Govt waives import duty on these Raw Materials and APIs
NPPA seeks details for 29 formulations from Drug Mfrs to fix…
Serum Institute to start Mfg Sputnik vaccine in September
Nutraceutical Industry: A trending Market
6 reasons: How Gel creams are different from traditional cold creams
FDA seizes fake drugs worth Rs. 50 lakh from drug dealers








