Last Updated on October 17, 2024 by The Health Master
Govt may soon allow import of untested drugs under trial
New Delhi: The Centre proposes to ease rules to allow the import of any new, untested drug undergoing clinical trials in other countries, for ‘compassionate use,’ such as those for Covid-19, rare paediatric disorders and genetic diseases.
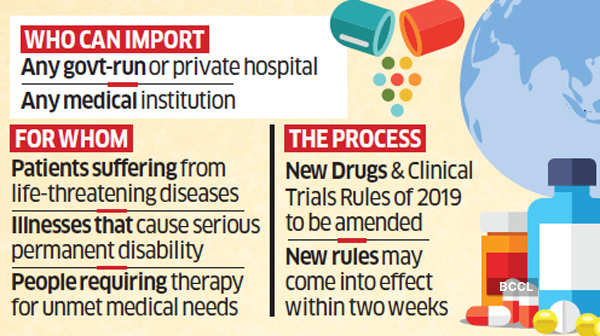
The ministry of health and family welfare is amending the New Drugs and Clinical Trials Rules 2019, enabling any government or private hospital, or a medical institution to import medicines required for the treatment of patients suffering from life-threatening diseases, those that cause serious permanent disability or people requiring therapy for unmet medical needs. The rules are likely to come into effect within two weeks, said people with knowledge of the matter.
This will help in the treatment of critically ill Covid-19 patients as it will give them access to drugs that may be unavailable in India and are undergoing clinical trials in other countries.
Also read: New Drugs and Clinical Trials draft Rules: Ministry
Process for Approvals
In clinical terms, ‘compassionate use’ is defined as the use of a new, unapproved drug to treat a seriously ill patient when no other therapy is available.
The process for approvals has been made simple, officials said. The medical institution will have to apply to the Drug Controller General of India (DCGI) explaining the rationale for ‘compassionate use’ over ‘available therapeutic options.’ Also required will be a description of the patient’s disease, criteria for selection of the patient and pharmacology and toxicology information adequate to conclude that the new drug is reasonably safe at the dose and duration proposed for compassionate use. The application will need to be addressed within 30 days. The draft amendment says: “The central licensing authority may, after scrutiny of information and documents enclosed with the application… if not satisfied with the requirements… reject the application, for reasons to be recorded in writing, within a period of 30 days from the date of application.”
The Centre had notified the New Drugs and Clinical Trials Rules last year to codify regulations for trials. However, the need for an amendment was felt because of the Covid-19 pandemic to expedite the import of new drugs available in other countries.
“The clause has been added to help patients suffering from serious diseases. This gives early access to patients suffering from rare diseases, especially children with spinal muscular atrophy or orphan diseases,” said a Central Drugs Standard Control Organisation (CDSCO) official. “This has been introduced as it also provides a safety net to doctors prescribing these medicines.” The medicines are expensive and are manufactured sparingly by drug manufacturers, sometimes costing over ₹1 crore per vial.
If a hospital prescribes a new drug unavailable in India, a manufacturer could also apply for a special licence and manufacture it in limited quantities. The manufacturer would need to get consent from the patient or legal heirs and approval from the ethics committee of the hospital. DCGI will then award a licence to manufacture the drug in limited quantities.
Remdesivir is being used in Europe under the ‘compassionate use’ clause to treat Covid-19. So far, India had no provision for such ‘compassionate use’ of drugs. “During Covid-19, America has made some drugs available to countries like Japan under ‘compassionate use’ clause,” said the official cited above.
“Where any medical officer of a hospital or medical institution prescribes a new drug for compassionate use for treatment of patients… such new drug may be approved to be manufactured in limited quantity subject to provisions of these rules,” according to the draft amendment.
Orphan diseases are rare illnesses that are little researched because of the lack of a big enough market.
The Health Master is now on Telegram. For latest update on health and Pharmaceuticals, subscribe to The Health Master on Telegram.


