- Key Notes on Revised Schedule M: Plant and equipment - August 16, 2025
- Drug Imports: New Online System for Dual Use NOC launched - August 2, 2025
- Amendment: Know About the Cosmetics (Amendment) Rules, 2025 - July 31, 2025
Last Updated on November 27, 2024 by The Health Master
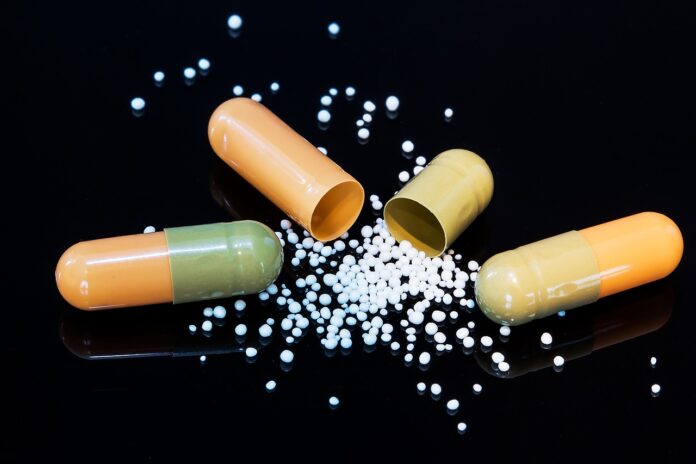
Granules
In the ever-evolving landscape of the pharmaceutical industry, the manufacturing processes have undergone significant advancements to meet the growing demands for quality medications. One such crucial advancement is the production of granules.
Granules, small particles formed from powder materials, play a pivotal role in the pharmaceutical sector due to their numerous benefits.
In this article, we will delve into the reasons, procedures, and functions behind granules manufacturing, shedding light on its vital contribution to the pharmaceutical world.
Why Granules? Understanding the Reasons Behind Granules Manufacturing
Granule manufacturing has gained immense popularity in the pharmaceutical industry for several compelling reasons:
- Enhanced Stability and Durability: Granules provide improved stability to delicate drug compounds, shielding them from environmental factors such as humidity and light, which can compromise the drug’s efficacy.
- Controlled Release: By creating granules with specific properties, pharmaceutical companies can achieve controlled release formulations, ensuring a gradual and sustained release of the active pharmaceutical ingredient (API) within the body.
- Homogeneous Mixing: Granules allow for uniform distribution of APIs and excipients, resulting in consistent dosing and reliable drug performance.
- Improved Solubility: Granulation processes can enhance the solubility of poorly soluble APIs, thus improving their bioavailability and overall effectiveness.
- Ease of Handling: Granules are easier to handle during manufacturing, packaging, and dispensing due to their uniform size and flow characteristics.
- Reduced Dust Formation: Compared to fine powders, granules produce less dust, minimizing the risk of cross-contamination and ensuring a safer working environment for employees.
Granules Manufacturing Procedures: From Raw Materials to Finished Product
The manufacturing of pharmaceutical granules involves several key procedures:
- Formulation Development: A comprehensive formulation is designed, considering factors such as API properties, desired release profiles, and compatibility with excipients.
- Wet Granulation or Dry Granulation: Two primary granulation techniques are employed. Wet granulation involves blending APIs and excipients, adding a liquid binder, and agglomerating the mixture into granules. Dry granulation, on the other hand, compacts the powder mixture into large granules without using liquid binders.
- Milling: In some cases, the granules are milled to achieve the desired particle size distribution and improve flow properties.
- Sieving: Granules are sieved to remove any oversized or undersized particles, ensuring uniformity.
- Blending: If necessary, different granules batches may be blended to achieve consistent drug content.
- Tableting or Capsule Filling: The final granules can be compressed into tablets or filled into capsules for convenient dosing.
Functions of Granules in the Pharmaceutical Industry
Granules serve several crucial functions that contribute to the efficacy and quality of pharmaceutical products:
- Improved Drug Stability: Granules shield APIs from environmental factors, preserving their potency over time.
- Enhanced Bioavailability: Granulation processes can enhance the solubility and dissolution rate of APIs, leading to better absorption in the body.
- Customized Formulations: Granules allow for the development of tailored formulations, catering to specific patient needs and treatment requirements.
- Precise Dosing: The uniformity of granules ensures accurate dosing, minimizing the risk of under- or overdosing.
- Reduced Side Effects: Controlled release granules can reduce the frequency of dosing, leading to fewer fluctuations in drug levels and potentially fewer side effects.
- Versatility: Granules can be used to create various dosage forms, from tablets and capsules to suspensions and sachets.
In conclusion, in the intricate realm of pharmaceuticals, the manufacturing of granules has emerged as a revolutionary process that addresses multiple challenges and elevates drug development.
With benefits ranging from enhanced stability and solubility to improved dosing precision, granules stand as a testament to the industry’s relentless pursuit of innovation.
As pharmaceutical companies continue to refine their manufacturing techniques, the role of granules is poised to grow even more significant, shaping the future of drug delivery and patient care.
by:
Rakesh Dahiya, SDCO cum Licensing Authority, FDA Haryana
Compiled by:
Rakesh Dahiya, ASDC, FDA Haryana
Guidelines for Export NOC revised
Apply WHO-GMP & CoPPs online: Step by step procedure
CDSCO Guidelines: Safe Disposal of Expired and Unused Drugs
Drugs and Cosmetics (Compounding of Offences) Rules, 2025: Gist
Qualification and Experience: For Technical Staff in Medical Devices
Key Notes on Revised Schedule M: Must read
Key Changes for Homoeopathic Medicines: GSR 669(E) dated 28-10-2024
Significance of Granules Manufacturing in the Pharmaceutical Industry
Gist of the draft notification: Compounding of Offences under the Drugs and Cosmetics Act
Laboratory Waste Management, Types and Its Disposal
How to Become a Skilled Manufacturing Chemist in the Pharma Industry
Sweet Science: Exploring Importance of Inverted Sugar in Pharma Industry
NABL certification for Laboratories: Let’s understand
Major FDA audit findings about Equipment and Instruments
Equipment and Instruments: Maintenance, difference and importance in the Pharma Industry
Quality Assurance in the Pharmaceutical Industry
NIPER: A Comprehensive Guide to NIPER Institutes in India
Latest on Indian Pharmacopoeia Commission (IPC)
Types of inspections done by USFDA: Read in detail
AHUs, Air Types, Air Changes and their Functions in Pharma Industry
Understanding Airlock Systems in Pharma Industry
Upcoming events: Pharma, Cosmetics, Homoeopathy & Medical Devices
Guidance documents for industry
Cosmetics Testing in India: A Comprehensive Guide
Airlocks in the Pharma Industry: An essential component
Line clearance and maintenance in manufacturing: A comprehensive guide
Difference between Validation and Calibration in the Pharma Industry
The Effectiveness of 70% Alcohol as a Disinfectant
Wet Granulation vs Dry Granulation: Understanding the Key Differences
Understanding GMP, cGMP, and WHO-GMP
USFDA issues Form 483 to Pharma Companies: Let’s know all about it
Calibration of Laboratory Instruments
Difference: Disintegration and Dissolution test in pharma industry
How to prepare SOPs in the Pharma Industry
Difference between branded and generic medicines
Understanding DQ, IQ, PQ, and OQ in the Pharma Industry
Duties and responsibilities of QA person in Pharma Industry
Types of Tablet Coating and its Functionality in pharma industry
Dissolution test: Importance in Pharma Industry
Lux level in Industry (Pharma, Cosmetics, Homeopathy & Medical Devices)
NSQ Drug: Route cause analysis and CAPA
Area required for manufacturing of Drugs, Cosmetics, Homoeopathic & Blood Centre