Last Updated on May 29, 2022 by The Health Master
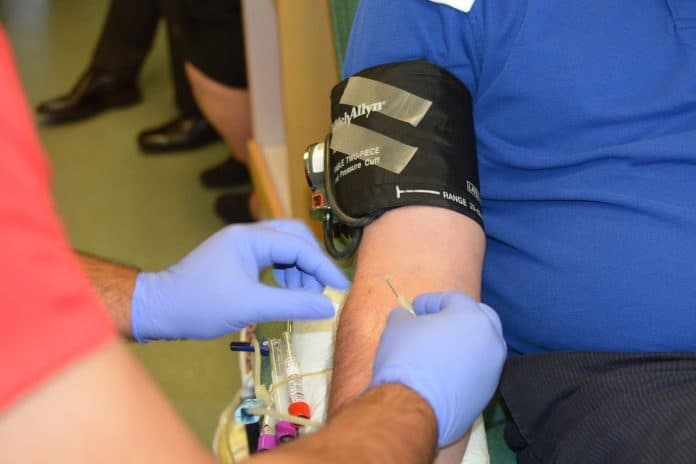
Nagpur: Four children in Nagpur became Human Immunodeficiency Virus (HIV) infection positive, allegedly after blood transfusion, said officials on Thursday.
These four patients were given blood for the treatment of Thalassemia, post which they were found infected.
Taking the matter into consideration, the state health department set up a high-level inquiry.
“Four children have been infected with HIV, out of which one child has died. We will collect all the information and take action against the culprits after conducting a high level investigation,” said Assistant Deputy Director of Health Department Dr RK Dhakate.
He said the Food and Drugs Department (FDA) has also started preliminary investigation in the case.
“(FDA) has also started preliminary investigation in this case. Nucleic Acid Test (NAT) test of blood given to Thalassemia patients would be done soon.
The doctor treating these patients said tainted blood was supplied to patients.
“They were tested during treatment and found HIV infected. Allegedly, they were infected with HIV and Hepatitis B after the contaminated blood was given to them by the blood bank.
It is necessary to have NAT test of blood given to the children suffering from Thalassemia, but due to non-availability of this facility in the blood bank, the children became victims of HIV infection,” said Dr Vicky Rughwani.
Earlier, five Thalassemic children were infected by Hepatitis C while two children were reportedly infected with Hepatitis B.
Further details are awaited.
Procedure to obtain license for Blood Centre (Blood Bank)
Latest Notifications: Blood Centre / Bank
Blood Centre (Bank) – requirements at a glance
FAQs – on Blood Bank / Centre (Part-1)
FAQs – on Blood Bank / Centre (Part-2)
FAQs – on Blood Bank / Centre (Part-3)
FAQs – on Blood Bags and its Testing
Mandatory requirements for Blood donation camps
FAQs on Legal Metrology & Blood Bags
Pharmacopoeial status of Blood and its components
Your Guide to Preventing and Treating Blood Clots
Adequate and safe blood transfusion for all: Article
Why India needs more blood donors: Article
USFDA lifts hold on Covaxin’s clinical trials
Govt allows import of Oxytocin Reference Standards for test & analysis
USFDA issues final approval to Alembic for Pirfenidone tablets
Hyderabad Pharma City to be part of new master plan
These important medical drugs are found in plants
De-addiction center raided by joint team of FDA Haryana & Police: HM
Drug recall: Over 10,000 bottles of generic Anti-depression Drug recalled
Financial aid to the family of late Sh. Sudip Ganguly, Drug Inspector West Bengal
Govt adds psoriasis drug Acitretin under Schedule H of Drugs Rules
Latest Notifications regarding Pharmaceuticals