Last Updated on June 25, 2022 by The Health Master
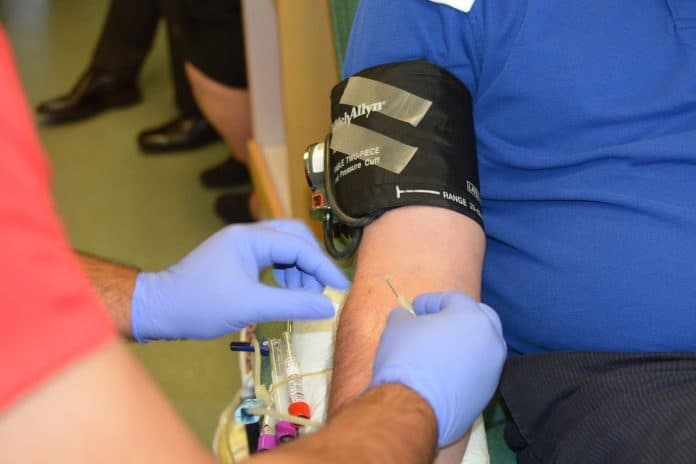
Gurugram: In a first for the state, the National Cancer Institute (NCI) in Jhajjar said it has adopted a new technology that helps in the early detection of viruses and reduces the risk of transmitting infections for patients who need blood transfusions.
Nucleic Acid Testing (NAT) is a molecular technique used for screening blood donations.
It can detect early-stage infectious agents such as HIV, hepatitis-C, and hepatitis-B by looking at genetic markers of viruses (DNA and RNA), making it a highly sensitive tool.
Normally, blood screening for viruses is done through serological tests that search for antibodies. These sometimes may not show up in the early stages of an infection.
Through analysis and imagination, design thinking empowers organisations to identify and implement humans.
Experts said the prevalence of HIV in blood donors is 0.5%. In the general population, the prevalence of hepatitis B is between 2% and 8%, and for hepatitis C, it is about 2%.
So far, Madhya Pradesh, Jammu & Kashmir and Odisha have synced NAT with all blood banks in the states. Elsewhere, the technology has been adopted by the AIIMS-Delhi and the Christian Medical College-Vellore.
Dr Diptiranjan Rout, assistant professor at the department of transfusion medicine, AIIMS in NCI Jhajjar, spoke about the risk of spreading infections through transfusions.
“The risk of any of these infections is multiplied three-four times as the single unit (of blood donated) is broken into three or four components, and transfused into patients depending on their need,” he said.
On adoption of NAT, he said: “NAT detects early-stage infectious agents and helps maximise safety. All health centres conducting blood transfusion should ideally adopt this.”
The doctor said the antibodies for infection are normally detected in serological (blood) tests about 20 to 80 days after a virus invades.
With NAT, this time period is reduced to 3-20 days. He added that NCI is the first facility in Haryana to incorporate this technology.
Procedure to obtain license for Blood Centre (Blood Bank)
Latest Notifications: Blood Centre / Bank
Blood Centre (Bank) – requirements at a glance
FAQs – on Blood Bank / Centre (Part-1)
FAQs – on Blood Bank / Centre (Part-2)
FAQs – on Blood Bank / Centre (Part-3)
FAQs – on Blood Bags and its Testing
Mandatory requirements for Blood donation camps
FAQs on Legal Metrology & Blood Bags
Pharmacopoeial status of Blood and its components
Your Guide to Preventing and Treating Blood Clots
Adequate and safe blood transfusion for all: Article
Why India needs more blood donors: Article
Blood Centre: Precautions and safety to be observed during blood transfusion
Blood Bank / Centre: Difference Between SDP and RDP
Haryana became the first state in the country to issue Drug Manufacturing Licenses online: HM
Govt notifies Surrogacy (Regulation) Rules for Surrogacy Clinics
UAFDA approves first Alopecia Drug that restores Hair Growth
Govt told to decide affordable price of Cancer Drug
Govt told to decide affordable price of Cancer Drug
Now TDS On Free Physician Samples
High Court to PCI: Open its portal for Pharmacy Colleges to submit application
Blood Bank / Centre: Difference Between SDP and RDP
Govt relaxes dossier filing norm for Medical Devices
Latest Notifications regarding Pharmaceuticals
For informative videos on Hospitals / Doctors, click on the below YouTube icon: